distinguish between a gravimetric and volumetric method of analysis|gravimetric vs volumetric water content : wholesaler The concentration is then calculated based on the mass of the precipitate and the stoichiometry of the reaction. Volumetric analysis, on the other hand, is a method of quantitative analysis that . Vagas abertas da Empresa VESTCASA. Junte-se à nossa equipe! ACREDITAR QUE É POSSÍVEL, É FAZER PARTE DO TIME VESTCASA! Com mais de 100 Lojas espalhadas pelo Brasil e com 15 anos de existência, a Vestcasa tem como objetivo democratizar o acesso dos mais variados itens para sua casa e seu dia a dia, passando por Utilidades .
{plog:ftitle_list}
Resultado da Neville Goddard wirkte über einen Zeitraum von 34 Jahren. In den 1950er Jahren ließ er sich in Los Angeles nieder, wo er eine Reihe von öffentlichen Vorträgen über die Methoden zum Gesetz der Annahme hielt. Zudem hielt er regelmäßig Vorträge im berühmten Wilshire Ebell Theater. Neville .
While gravimetric analysis relies on the measurement of mass and the formation of a precipitate, volumetric analysis involves the measurement of volume and the reaction between the . Gravimetric and Volumetric Analysis are common methods used in analytical chemistry. They provide mechanism for determining the amounts of substances present in a . Gravimetric Analysis refers to a quantitative method in which the amount of an analyte is determined based on its mass. On the other hand, Volumetric Analysis involves determining the quantity of an analyte by .The concentration is then calculated based on the mass of the precipitate and the stoichiometry of the reaction. Volumetric analysis, on the other hand, is a method of quantitative analysis that .
Differences between volumetric analysis and gravimetric analysis. In contrast to volumetric analysis, which determines the amount of a desired element by measuring its volume, gravimetric analysis measures the amount .
Principles of Volumetric Analysis Volumetric analysis is a method to determine the amount of substances in a given sample. A procedure called titration is used in volumetric analysis. In . An accurate gravimetric analysis requires that the analytical signal—whether it is a mass or a change in mass—is proportional to the amount of analyte in our sample. For all gravimetric methods this proportionality . Gravimetry includes all analytical methods in which the analytical signal is a measurement of mass or a change in mass. When you step on a scale after exercising you . This difference highlights the contrast between the two methods: gravimetric focuses on mass changes, and volumetric focuses on volume changes. 13 The accuracy of gravimetric analysis often depends on the .
Define and distinguish between acids and bases. Distinguish between monoprotic and polyprotic acid-base equilibrium. Describe and distinguish between weak acid/base dissociations. Have a working . Before we consider specific gravimetric methods, let’s take a moment to develop a broad survey of gravimetry. . The mass of suspended solids is the difference between the filter’s final mass and its original mass. . Gravimetric analysis is an analytical technique used for the quantitative determination of an analyte based on the mass of a solid. . Electro gravimetric method is employed to separate the ions of a substance, often a metal. In this method, the analyte solution is electrolyzed. . Differences between volumetric analysis and gravimetric analysis. Gravimetric analysis is an uncomplicated, easy-to-follow laboratory method that helps in the quantitative determination of chemical compounds. It is based on measuring the amount of a chemical compound depending upon its loss in mass. How is gravimetric analysis different from a volumetric analysis method such as titration?
digital compression testing machine 3000kn
Gravimetric analysis is a quantitative method used in analytical chemistry to determine the amount of a substance present in a sample by measuring its mass. This technique relies on the principles of precipitation and weighing to isolate and quantify the analyte of interest. . In this process, a known volume of the sample solution containing .Principles of Volumetric Analysis Volumetric analysis is a method to determine the amount of substances in a given sample. A procedure called titration is used in volumetric analysis. In titration, a solution of known concentration, called a standard solution, is added to a measured volume of an unknown solution until the reaction is completed. Gravimetric Analysis refers to a quantitative method in which the amount of an analyte is determined based on its mass. On the other hand, Volumetric Analysis involves determining the quantity of an analyte by measuring its volume.
Gravimetric analysis describes a set of methods used in analytical chemistry for the quantitative determination of an analyte (the ion being analyzed) based on its mass. The principle of this type of analysis is that once an ion's mass has been determined as a unique compound, that known measurement can then be used to determine the same analyte's mass in a mixture, as long as .The availability of commercial gravimetric and volumetric systems for the measurement of adsorption equilibrium has seen also a growth of the use of these instruments to measure adsorption kinetics. A review of publications from the past 20 years has been used to assess common practice in 180 cases. There are worrying trends observed, such as lack of .Gravimetric analysis describes a set of methods used in analytical chemistry for the quantitative determination of an analyte (the ion being analyzed) based on its mass. . The difference between the filter's original mass and final mass gives the mass of suspended solids. It is a direct analysis because the analyte itself is the object being .
The steps involved in a gravimetric analysis differ slightly between the methods used. However, in all methods, something is weighed, and the mass of an analyte is determined.
Because the release of a volatile species is an essential part of these methods, we classify them collectively as volatilization gravimetric methods of analysis. 8.4: Particulate Gravimetry Precipitation and volatilization gravimetric methods require that the analyte, or some other species in the sample, participates in a chemical reaction.Gravimetric Analysis. A gravimetric analysis is one in which a sample is subjected to some treatment that causes a change in the physical state of the analyte that permits its separation from the other components of the sample. Mass measurements of the sample, the isolated analyte, or some other component of the analysis system, used along with the known stoichiometry of .Difference between Gravimetric and Volumetric analysis: Practice Problems: Frequently Asked Questions -FAQs: Gravimetric Analysis: Gravimetric analysis is one of the commonly used quantitative methods in analytical .Before we consider specific gravimetric methods, let’s take a moment to develop a broad survey of gravimetry. . The mass of suspended solids is the difference between the filter’s final mass and its original mass. . An accurate gravimetric analysis requires that the analytical signal—whether it is a mass or a change in mass—be .
Q: What is the difference between gravimetric and volumetric analysis? A: Gravimetric analysis and volumetric analysis are two different methods used in quantitative analysis. The gravimetric analysis involves . Gravimetric Analysis. Gravimetric analysis is a quantitative method in analytical chemistry wherein the concentration of a substance present in a sample is evaluated based on the measurement of its mass. This is done by precipitating the analyte (substance being analyzed) in a sample as a solid compound which is then separated, washed, dried and weighed. Gravimetric analysis and volumetric analysis are both analytical techniques used in chemistry to determine the quantity or concentration of a substance in a sample. However, they differ in their methods and principles. Step 2/3 1. Gravimetric analysis: - Gravimetric analysis is based on the measurement of the mass of a substance.
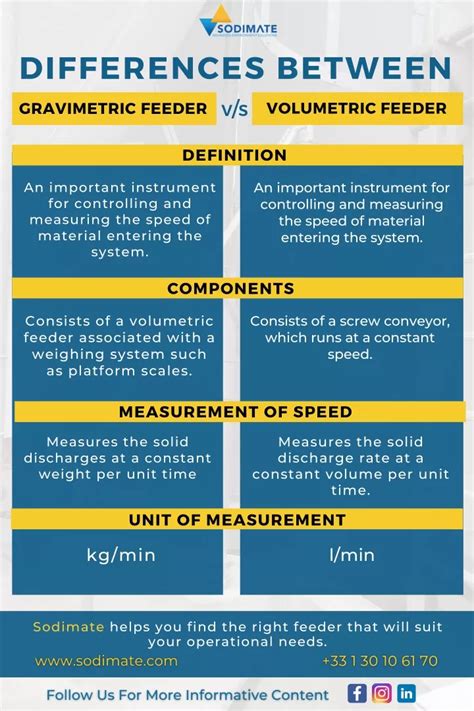
volumetric vs gravimetric feeder
volumetric and gravimetric analysis
suitable method and requirements for gravimetric Outline the difference between nucleation, precipitate th and define the concentration parameters Recognize the meaning of indicators and identify the suitable condition of gravimetric analysis and removal of contamination Describe statistical methods in analytical chemistry.Learn about gravimetric analysis, a method to determine the amount of a substance by measuring its mass.The first method of Volumetric Analysis was devised and found by the French chemist Jean-Baptiste-Andre-Dumas; as he was trying to determine the proportion of nitrogen combined with other elements in organic compounds. . Based on the desired endpoint, single drops or less than a drop of the titrant makes a difference between a permanent and .
Titrations play a crucial role in quality assessment of inorganic chemicals and are still routinely done volumetrically with visual endpoint detection. These procedures require a significant amount of glassware, e.g., burettes designated for specific volumetric solutions, pipettes, volumetric flasks, the use of which presents many drawbacks such as liquid thermal .volume Overview Students collect soil samples with a trowel or auger and weigh them, dry them, and then weigh them again. Students will also measure the volume of the collection container. The soil water content is determined by calculating the difference between the wet sample mass and the dry sample mass. Student Outcomes
Volumetric Analysis: Gravimetric Analysis: It is a quantitative analysis method where the amount of substance can be known by examining its volume. It is a quantitative analysis used to determine the weight of an unknown compound in a sample. The volumetric analysis provides the amount of compound in the units of volume like L (litres) and mL.Gravimetric analysis is a method of a quantitative assessment of laboratory techniques based mostly on the dimension of an analyte's mass. One example of a gravimetric evaluation technique may be used to decide the quantity of an ion in an answer by way of dissolving a regarded quantity of a compound containing the ion in a solvent to break up the ion from its .
In most cases the precipitate is the product of a simple metathesis reaction between the analyte and the precipitant; however, any reaction that generates a precipitate potentially can serve as a gravimetric method. Most precipitation gravimetric methods were developed in the nineteenth century, or earlier, often for the analysis of ores .
digital compression testing machine capacity: 2000 kn
WEBFodendo essa morena gostosa de quatro. Video porno vazado da Catarina Paolino transando pela primeira vez. Assistir video de sexo da youtuber Catarina fodendo .
distinguish between a gravimetric and volumetric method of analysis|gravimetric vs volumetric water content